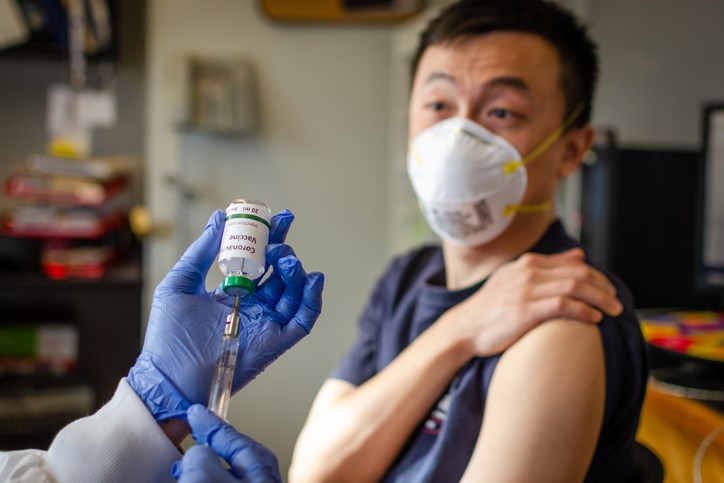
A vaccine against COVID-19 currently undergoing human trials in China could soon see clinical trials in Canada too.
That’s according to a May 12 press release from the National Research Council of Canada (NRC), which announced collaboration with the Chinese research group today to “advance bioprocessing and clinical development of the candidate vaccine in Canada.
“This vaccine candidate holds great promise. Until such time as there is an effective vaccine for COVID-19, the virus will continue to disrupt all aspects of our society and economy,” said NRC president Iain Stewart in the release.
Using technology developed in Canada and China, it was the first candidate vaccine in the world to make it to Phase 2 human clinical trials. However, before any trials begin in Canada, the vaccine would need approval from Health Canada.
One of over 100 coronavirus vaccines in development around the world, CanSino Biologics Inc. received Chinese regulatory approval for the Ad5-nCoV vaccine in March.
This is not the first time the NRC has collaborated with the Chinese research group. In 2013, the NRC licensed a cell liner to the company used in the development of a vaccine against the Ebola virus.
Research groups across the world are working on vaccines against COVID-19 at speeds never seen before, as generally, vaccines take between five and 15 years to develop, according to the NRC.
B.C.’s four-phase plan to reduce restrictions and re-open the economy begins with the resumption of elective surgeries, personal care services – such as dentistry and hair salons. Retail and the reopening of provincial parks for day use starts May 19.
But concerts, conventions, large professional sporting events and other large gatherings — phase four of B.C.'s restart plan — could remain banned for one to two years, with their resumption contingent on a vaccine being developed, the development of herd immunity or a new drug to treat the COVID-19 virus.
The CanSino Biologics Inc. candidate vaccine isn’t the only one to barrel ahead in its development.
Also on Tuesday, a research group out of Cambridge, Mass., known as Modern Therapeutics, received fast-track approval from the U.S. Food and Drug Administration for its COVID-19 vaccine candidate, only days after receiving approval to proceed with Phase 2 testing.
— with files from Nelson Bennett and Hayley Woodin